Polyphenols
Polyphenols are natural compounds found in fruits, vegetables, cereals, and drinks like tea and coffee. For example, fruits such as grapes, apples, pears, cherries, and berries contain around 200-300 mg of polyphenols per 100 grams. Products made from these fruits also have significant amounts of polyphenols. A glass of red wine or a cup of tea or coffee typically has about 100 mg of polyphenols. Cereals, legumes, and chocolate also add to our polyphenol intake (Scalbert et al., 2005; Spencer et al., 2008).
Plants produce polyphenols as a kind of defense against UV radiation and attacks from pathogens. In food, polyphenols affect bitterness, astringency, color, flavor, smell, and how well the food resists spoiling. Studies since the late 20th century suggested that diets rich in plant polyphenols might protect against cancers, heart diseases, diabetes, osteoporosis, and neurodegenerative diseases (Graf et al., 2005; Arts et al., 2005). This has sparked a lot of interest in the scientific community about the potential health benefits of polyphenols and their role in preventing diseases.
As we will see below, the fermentation process also enable the synthesis and stabilization of polyphenolic compounds, contributing to the increase of antioxidant activity of fermented products.
Structure and Classes of Polyphenols
More than 8,000 polyphenolic compounds have been identified in various plant species. These compounds all originate from a shared starting point, either phenylalanine or a closely related substance called shikimic acid. Typically, these compounds are found in combined forms, with one or more sugar units attached to hydroxyl groups. However, there are also cases where sugars are directly linked to an aromatic carbon. Additionally, polyphenols often associate with other compounds like carboxylic and organic acids, amines, lipids, and other phenols (Kondratyuk, at al., 2004).
Polyphenols can be categorized based on the number of phenol rings they contain and the structural elements that connect these rings. The main classes include phenolic acids, flavonoids, stilbenes, and lignans (Spencer at al., 2008). The figure below provides a visual representation of these polyphenol groups and their chemical structures.
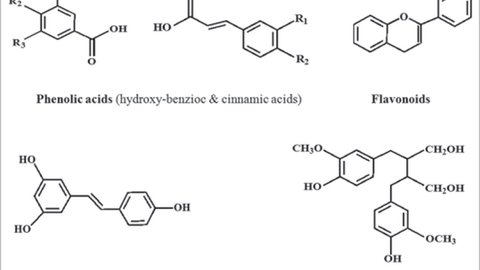
Phenolic Acids
Phenolic acids are widely present in various foods and can be classified into two main groups: those derived from benzoic acid and those derived from cinnamic acid. The levels of hydroxybenzoic acid in edible plants are generally modest, except in specific cases like certain red fruits, black radish, and onions, where concentrations can reach several tens of milligrams per kilogram of fresh weight (Shahidi at al, 1995). On the other hand, hydroxycinnamic acids are more prevalent than hydroxybenzoic acids and mainly include p-coumaric, caffeic, ferulic, and sinapic acids (Pandey et al., 2009).
Flavonoids
Flavonoids represent the most extensively researched category of polyphenols. This group shares a common foundational structure, consisting of two aromatic rings connected by three carbon atoms that create an oxygenated heterocycle. With over 4,000 identified varieties, many flavonoids contribute to the colors observed in flowers, fruits, and leaves. Their classification into six subclasses—flavonols, flavones, flavanones, flavanols, anthocyanins, and isoflavones (refer to the figure below) is based on the type of heterocycle involved. Differences within each subclass stem from variations in the number and arrangement of hydroxyl groups, as well as the degree of alkylation and/or glycosylation (Spencer et al., 2008). Common flavonoids include quercetin, myricetin, and catechins.
Stilbenes
Stilbenes are a group of compounds found in various plant species, including grapes, peanuts, berries and several medicinal plants, known for their diverse biological activities. These activities include anti-tumor, anti-inflammatory, antioxidant, antibacterial, and antiviral properties (Xin et al., 2022). Research has shown that stilbenes have potential benefits for human health, such as neuroprotective and anti-obesity effects, and they have been studied for their potential role in the prevention and treatment of cancer (Sirerol et al., 2015; Reinisalo et al., 2015; Benbouguerra et al., 2021).
Stilbenes have been shown to modulate various cellular processes involved in inflammation and have been identified as a versatile class of natural metabolites for inflammation (Al-Khayri et al., 2023).
However, the bioactivity of natural stilbenes in humans is still not fully understood, and more rigorous studies are needed to determine their effectiveness and establish standardized dietary/therapeutic dosages (Sirerol et al., 2015).
Stilbenes contain two phenyl moieties connected by a two-carbon methylene bridge. Occurrence of stilbenes in the human diet is quite low. Most stilbenes in plants act as antifungal phytoalexins, compounds that are synthesized only in response to infection or injury. One of the best studied, naturally occurring polyphenol stilbene is resveratrol (3,4′,5-trihydroxystilbene), found largely in grapes (Pandey et al., 2009).
Lignans
Lignans are diphenolic compounds that contain a 2,3-dibenzylbutane structure that is formed by the dimerization of two cinnamic acid residues. Several lignans, such as secoisolariciresinol, are considered to be phytoestrogens (Adlercreutz at al., 1997).
Lignans possess a steroid-like chemical structure and are considered to be phytoestrogens. They are bioactive compounds exhibiting various biological properties, including anti-inflammatory, antioxidant, and antitumor activities. Lignans are found in relatively low concentrations in various seeds, grains, fruits, and vegetables, and in higher concentrations in sesame and flax seeds. The level of lignan ingestion, and thus lignan bioavailability, depends on the type of diet consumed and can be highly variable. After ingestion, plant lignans are metabolized by intestinal bacteria, undergoing transformation to mammalian lignans prior to absorption. This apparently considerably decreases the risk of diverse types of cancer, particularly of the colon, prostate, and breast. Dietary intake of lignan-rich foods could be a useful way to bolster the prevention of chronic illness, such as certain types of cancers and cardiovascular disease (Rodríguez-García at al, 2019).
Distribution and storage effects
The distribution of phenolics in plants varies at different levels—tissues, cells, and subcellular structures. Insoluble phenolics are found in cell walls, while soluble ones are located in plant cell vacuoles. Polyphenols like quercetin are widespread in various plant products, while flavanones and isoflavones are specific to certain foods. Plant foods generally contain complex mixtures of polyphenols, with higher levels in outer plant layers than inner parts (Simon et al., 1992).
Various factors influence polyphenol content, such as ripeness, environmental conditions, and processing. Ripeness significantly affects polyphenol concentrations, with phenolic acid content decreasing and anthocyanin concentrations increasing during ripening. Polyphenols, particularly phenolic acids, play a direct role in plant responses to stress, contributing to healing and possessing antimicrobial properties (Manach et al.,2004; Parr et al., 2000).
Storage also affects polyphenol content due to oxidation reactions. Foods may undergo changes in color and taste, which can be beneficial (e.g., black tea) or harmful (e.g., browning of fruit). The polyphenolic content of stored wheat flour decreases, with a 70% reduction after six months. Cold storage has a minor impact on polyphenols in apples, pears, or onions.
Cooking alters polyphenol concentrations significantly. Onions and tomatoes lose 75-80% of quercetin content after boiling for 15 minutes, 65% after microwave cooking, and 30% after frying (Sosulski et al, 1982; Price et al., 1997).
Bioavailability of Polyphenols
Bioavailability refers to how much of a nutrient is digested, absorbed, and metabolized through normal pathways in the body. Each polyphenol, a type of antioxidant-rich compound found in plants, has its own bioavailability, and the amount of polyphenols in food doesn’t necessarily correlate with how much the body can absorb (D’Archivio et al., 2007). Most polyphenols are present in foods as esters, glycosides, or polymers that need to be hydrolyzed by intestinal enzymes or colonic microflora before absorption.
During absorption, polyphenols undergo significant modifications, including conjugation in intestinal cells and later in the liver through processes like methylation, sulfation, and glucuronidation (Day et al., 2001). This results in different forms reaching the blood and tissues compared to those present in food, making it challenging to identify all metabolites and evaluate their biological activity (Setchell et al., 2003).
The chemical structure of polyphenols, not their concentration, determines absorption rates and the nature of circulating metabolites (Setchell et al., 2003). Polyphenols vary in their absorption sites within the human body. Some are well absorbed in the gastrointestinal tract, while others are absorbed in the intestine or other parts of the digestive tract.
Factors like glucosylation, polymeric nature, and high molecular weight affect the absorption of specific polyphenols (D’Archivio et al., 2007). Storage and cooking also impact polyphenol content, with oxidation reactions occurring during storage, and cooking methods affecting concentrations in foods.
After absorption, polyphenols undergo conjugation processes such as methylation, sulfation, and glucuronidation, facilitating their elimination through urine and bile (Day et al., 2001). Albumin, a protein in the blood, binds to polyphenol metabolites, affecting their bioavailability and potential biological activity (D’Archivio et al., 2007).
The accumulation of polyphenols in tissues, particularly those where they are metabolized, is crucial for their biological effects. Excretion of polyphenols and their derivatives occurs through urine and bile, and the health benefits of polyphenols depend on both intake and bioavailability (D’Archivio et al., 2007).
Health benefits
Polyphenols are micronutrients present in a variety of foods, which gained interest over the last 30 years due to their antioxidant properties and their emerging role in the prevention of several diseases linked to oxidative stress such as cancer, cardiovascular and neurodegenerative disorders (Scalbert et al., 2005).
Several studies, both clinical and experimental, have shown that including polyphenol-rich foods and drinks in diet can boost the antioxidant capacity in the bloodstream. These compounds also help reduce oxidative damage to DNA, possess anti-inflammatory properties, and modulate the immune system. These factors contribute to their role in protecting against cardiovascular diseases (Nakagawa et al., 1999; Rein atal., 2000; Leighton at al., 1999; Lotito et al., 2011).
The persistent use of polyphenols is clinically relevant in terms of the reduction of vascular risk factors for Ischemic stroke, such as Atrial Fibrillation. Interestingly, different kinds of polyphenols provide brain protection by activating different pathways and mechanisms, like inducing antithrombotic effect, such as Honokiol (Pacifici et al., 2021).
Polyphenols in fermentation
Acetic acid bacteria enables the synthesis and stabilization of polyphenolic compounds and antioxidant activity in fermented food and their impact on human health as shown in a systematic review study. Although large heterogenicity of methods and material used (Kombucha, vinegar, fermented cocoa beans, and sour beer), there is evidence of the action of acetic acid bacteria towards stabilization or increase of antioxidant activity of the fermented products (Katarzyna et al., 2023).
During the fermentation, microbials produce a large number of acids, alcohols, and esters. The way polyphenolic content increases or stays stable during this process might be linked to reactions like oxidation and hydrolysis. These reactions are related to a decrease in pH and the production of several enzymes, including β-glucosidase, esterase, cellulase, glucanase, xylanase, pectinase, and others. This process may cause large molecular compounds to break down into smaller polyphenol monomers.
In plant tissues like tea leaves, fruits, and vegetables, there are a lot of insoluble bound phenols that are hard to release into water. During fermentation, enzymes from acetic acid bacteria can break down these bound phenols, releasing them into the solution. Additionally, the low pH value during fermentation can help stabilize the polyphenolic compounds in the solution and increase their antioxidant activity (Noronha et al., 2022, Wang et al., 2022, Wang et al., 2022).
The acidification of the environment has a stabilizing effect on the oxidation reactions, which limits or even inhibits the enzymatic oxidation of polyphenolic compounds (Shahidi et al., 2015, Zhou et al., 2020). Therefore, fermented food products are a good source of polyphenolic compounds, including various types of liquid products resulting from oxidative fermentation, such as vinegar, wine, beer, or Kombucha tea drink (Katarzyna et al., 2023).
Section references
Scalbert A, Manach C, Morand C, Remesy C. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45:287–306.
Spencer JP, Abd El Mohsen MM, Minihane AM, Mathers JC. Biomarkers of the intake of dietary polyphenols: strengths, limitations and application in nutrition research. Br J Nutr. 2008;99:12–22.
Graf BA, Milbury PE, Blumberg JB. Flavonols, flavonones, flavanones and human health: Epidemological evidence. J Med Food. 2005;8:281–290
Arts ICW, Hollman PCH. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005;81:317–325.
Kondratyuk TP, Pezzuto JM. Natural Product Polyphenols of Relevance to Human Health. Pharm Biol. 2004;42:46–63.
Spencer JP, Abd El Mohsen MM, Minihane AM, Mathers JC. Biomarkers of the intake of dietary polyphenols: strengths, limitations and application in nutrition research. Br J Nutr. 2008;99:12–22.
Xin Su, Ning Li. Chapter 8 - Bioactive stilbenes from plants. Studies in Natural Products Chemistry. 2022
Sirerol JA, Rodríguez ML, Mena S, Asensi MA, Estrela JM, Ortega AL. Role of Natural Stilbenes in the Prevention of Cancer. Oxid Med Cell Longev. 2016;2016:3128951. doi: 10.1155/2016/3128951. Epub 2015 Dec 21. PMID: 26798416; PMCID: PMC4698548.
Reinisalo M, Kårlund A, Koskela A, Kaarniranta K, Karjalainen RO. Polyphenol Stilbenes: Molecular Mechanisms of Defence against Oxidative Stress and Aging-Related Diseases. Oxid Med Cell Longev. 2015;2015:340520. doi: 10.1155/2015/340520. Epub 2015 Jun 9. PMID: 26180583; PMCID: PMC4477219.
Al-Khayri, J.M.; Mascarenhas, R.; Harish, H.M.; Gowda, Y.; Lakshmaiah, V.V.; Nagella, P.; Al-Mssallem, M.Q.; Alessa, F.M.; Almaghasla, M.I.; Rezk, A.A.-S. Stilbenes, a Versatile Class of Natural Metabolites for Inflammation—An Overview. Molecules 2023, 28, 3786. https://doi.org/10.3390/molecules28093786
Benbouguerra N., Hornedo-Ortega, R., Garcia F.,El Khawand T., Saucier, C., Richard, T. Stilbenes in grape berries and wine and their potential role as anti-obesity agents: A review. Trends in Food Science & Technology. 2021
Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009 Nov-Dec;2(5):270-8. doi: 10.4161/oxim.2.5.9498. PMID: 20716914; PMCID: PMC2835915.
Shahidi F, Naczk M. Food phenolics, sources, chemistry, effects, applications. Lancaster, PA: Technomic Publishing Co Inc; 1995.
Adlercreutz H, Mazur W. Phyto-oestrogens and Western diseases. Ann Med. 1997;29:95–120.
Rodríguez-García C, Sánchez-Quesada C, Toledo E, Delgado-Rodríguez M, Gaforio JJ. Naturally Lignan-Rich Foods: A Dietary Tool for Health Promotion? Molecules. 2019 Mar 6;24(5):917. doi: 10.3390/molecules24050917. PMID: 30845651; PMCID: PMC6429205.
Simon BF, Perez-Ilzarbe J, Hernandez T, Gomez-Cordoves C, Estrella I. Importance of phenolic compounds for the characterization of fruit juices. J Agric Food Sci. 1992;40:1531–1535.
Manach C, Scalbert A, Morand C, Rémésy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747.
Parr AJ, Bolwell GP. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenol content or profile. J Agric Food Chem. 2000;80:985–1012.
Sosulski FW, Krygier K, Hogge L. Importance of phenolic compounds for the characterization of fruit juices. J Agric Food Chem. 1982;30:337–340.
Price KR, Bacon JR, Rhodes MJC. Effect of storage and domestic processing on the content and composition of flavonol glucosides in onion (Allium cepa) J Agric Food Chem. 1997;45:938–942.
Pacifici F, Rovella V, Pastore D, Bellia A, Abete P, Donadel G, Santini S, Beck H, Ricordi C, Daniele ND, Lauro D, Della-Morte D. Polyphenols and Ischemic Stroke: Insight into One of the Best Strategies for Prevention and Treatment. Nutrients. 2021 Jun 8;13(6):1967. doi: 10.3390/nu13061967. PMID: 34201106; PMCID: PMC8229516.
D’Archivio M, Filesi C, Benedetto RD, Gargiulo R, Giovannini C, Masella R. Polyphenols, dietary sources and bioavailability. Ann Ist Super Sanità 2007;43:348–361.
Day AJ, Williamson G. Biomarkers for exposure to dietary flavonoids: a review of the current evidence for identification of quercetin glycosides in plasma. Br J Nutr. 2001;86:S105–S110.
Setchell KD, Faughnan MS, Avades T, Zimmer-Nechemias L, Brown NM, et al. Comparing the pharmacokinetics of daidzein and genistein with the use of 13C-labeled tracers in premenopausal women. Am J Clin Nutr. 2003;77:411–419.
Scalbert A., Manach C., Morand C., Remesy C., Jimenez L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005;45:287–306. doi: 10.1080/1040869059096.
Nakagawa K., Ninomiya M., Okubo T., Aoi N., Juneja L.R., Kim M., Yamanaka K., Miyazawa T. Tea catechin supplementation increases antioxidant capacity and prevents phospholipid hydroperoxidation in plasma of humans. J. Agric. Food Chem. 1999;47:3967–3973. doi: 10.1021/jf981195l.
Rein D., Lotito S., Holt R.R., Keen C.L., Schmitz H.H., Fraga C.G. Epicatechin in human plasma: In vivo determination and effect of chocolate consumption on plasma oxidation status. J. Nutr. 2000;130:2109S–2114S. doi: 10.1093/jn/130.8.2109S.
Leighton F., Cuevas A., Guasch V., Perez D.D., Strobel P., San Martin A., Urzua U., Diez M.S., Foncea R., Castillo O., et al. Plasma polyphenols and antioxidants, oxidative DNA damage and endothelial function in a diet and wine intervention study in humans. Drugs Exp. Clin. Res. 1999;25:133–141. [PubMed] [Google Scholar]
Lotito S.B., Zhang W.J., Yang C.S., Crozier A., Frei B. Metabolic conversion of dietary flavonoids alters their anti-inflammatory and antioxidant properties. Free Radic. Biol. Med. 2011;51:454–463. doi: 10.1016/j.freeradbiomed.2011.04.032.
Katarzyna Neffe-Skocińska, Marcelina Karbowiak, Marcin Kruk, Danuta Kołożyn-Krajewska, Dorota Zielińska, Polyphenol and antioxidant properties of food obtained by the activity of acetic acid bacteria (AAB) – A systematic review. Journal of Functional Foods, Volume 107, 2023.
Noronha, M. C., Cardoso, R. R., dos Santos D’Almeida, C. T., Vieira do Carmo, M. A., Azevedo, L., Maltarollo, V. G., Júnior, J. I. R., Eller, M. R., Cameron, L. C., Ferreira, M. S. L., & Barros, F. A. R. de. (2022). Black tea kombucha: Physicochemical, microbiological and comprehensive phenolic profile changes during fermentation, and antimalarial activity. Food Chemistry, 384, 132515. https://doi.org/10.1016/j.foodchem.2022.132515.
D. Wang, M. Wang, L. Cao, X. Wang, J. Sun, J. Yuan, S. Gu. Changes and correlation of microorganism and flavor substances during persimmon vinegar fermentation. Food Bioscience, 46 (2022), Article 101565, 10.1016/j.fbio.2022.101565
X. Wang, D. Wang, H. Wang, S. Jiao, J. Wu, Y. Hou, J. Sun, J. Yuan. Chemical Profile and Antioxidant Capacity of Kombucha Tea by the Pure Cultured Kombucha. LWT, 168 (2022), Article 113931, 10.1016/j.lwt.2022.113931
F. Shahidi, P. Ambigaipalan. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects – A review. Journal of Functional Foods, 18 (2015), pp. 820-897, 10.1016/j.jff.2015.06.018
L. Zhou, T. Liao, W. Liu, L. Zou, C. Liu, N.S. Terefe. Inhibitory effects of organic acids on polyphenol oxidase: From model systems to food systems. Critical Reviews in Food Science and Nutrition, 60 (21) (2020), pp. 3594-3621, 10.1080/10408398.2019.1702500